The Strategy for the prioritisation of substances under HBM4EU was developed in the first half of 2017 and agreed at the meeting of the Governing Board in September 2017.
The strategy sets out the steps involved in identifying substances for research and surveys under the project. The Strategy also includes a set of prioritisation criteria, against which substances were assessed.
The prioritisation strategy was implemented from July 2017 to July 2018 during the 2nd round of prioritisation. The principle objective of the strategy was to generate the 2nd list of HBM4EU priority substances.
Broader objectives are presented here and the full process is documented in the project deliverable on the stakeholder consultation and mapping of needs.
The prioritisation strategy was implemented in two distinct tasks:
- the mapping of knowledge needs; and
- the prioritisation of substances.
The European Environment Agency (EEA) led implementation of the task on the mapping of knowledge needs, supported by the Austrian Environment Agency (EAA).
The French Agency for Food, Occupational Health and Safety (ANSES) led the task on the prioritisation of substances, supported by a team of experts from VITO and the German Environment Agency. In practice, all partners closely collaborated in delivering a coherent prioritisation strategy.
The figure below presents the breakdown of key steps in the process across these two tasks.
Overview of the prioritisation strategy
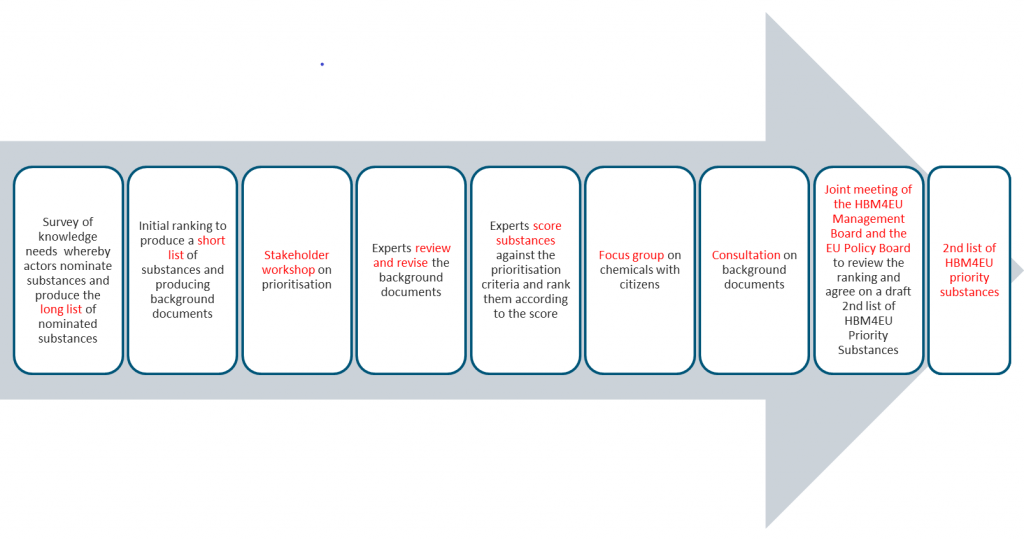
Click here to download the overview of prioritisation strategy.
Key steps in the prioritisation strategy are presented below. For additional details, please click on each step.
- Step 1: An online survey requesting the nomination of substances for research under HBM4EU in which members of the EU Policy Board, the National Hub Contact Points and the members of the Stakeholder Forum nominated substances and/or groups of substances for work under the project. Survey participants were asked to justify their nominations by submitting information against the prioritisation criteria.
- Step 2: Collation of survey results to produce a long list of all newly nominated substances and substance groups. This involved removing all re-nominations of substances on the 1st list of HBM4EU Priority Substances, as well as consolidating single substances and groups of substances where there was overlap.
- Step 3: Reducing the long list down to a short list of nominated substances and substance groups, by identifying those substances for which EU policy makers identified a need for evidence at EU level, and for which there was broad support.
- Step 4: Stakeholder workshop on prioritisation, allowing for an open discussion on substances on the short list and giving the HBM4EU partners a chance to learn about stakeholder priorities and concerns in greater detail.
- Step 5: Producing draft background documents on all substances on the short list by mapping the information submitted in the online survey against the five prioritisation criteria. Undertaking an expert review and revision of the background documents to include any missing information.
- Step 6: Scoring the substances on the short list against the prioritisation criteria and categorising substances according to the availability of human biomonitoring data.
- Step 7: Initial ranking of substances on the short list.
- Step 8: Consulting the members of the Stakeholder Forum, the EU Policy Board and the National Hubs on the background documents and scores. Reviewing and revising the background document and score for each substance and/or group according to the comments received. Final ranking of substances on the short list.
- Step 9: Joint meeting of the HBM4EU Management Board and the EU Policy Board to review the final ranking and agree on a draft 2nd list of HBM4EU priority substances, taking into account the results of the prioritisation strategy, available project resources and political priorities.
- Step 10: Approval of the final list of HBM4EU priority substances by the HBM4EU Governing Board.
In addition and with the explicit aim of understanding public concerns in order to complement the formal prioritisation strategy, HBM4EU undertook two outreach activities with European citizens. An online survey was conducted with European citizens on human biomonitoring. This was complemented by a focus group on chemicals held in Austria with members of the public. The results of these two outreach activities are summarised in a report on HBM4EU_outreach to European citizens and have been disseminated to the HBM4EU partners to inform our ongoing work.
Implementation of the steps began in 2017, with the aim of identify the 2nd list of HBM4EU priority substances. The Governing Board approved the 2nd list of HBM4EU priority substances in July 2018.
More information on this process may be found in the following deliverables:
- D4.3 Prioritisation strategy & criteria
- D4.4 First report on the stakeholder consultation and the mapping of needs
- D4.5 Second list of HBM4EU priority substances and CGLs
The 2nd list of HBM4EU priority substances includes:
- Acrylamide
- Aprotic solvents
- Arsenic
- Diisocyanates
- Lead
- Mercury
- Mycotoxins
- Pesticides
- UV filters – Benzophenones
Regarding the substance group on pesticides, HBM4EU partners and the Chemical Substance Group Leader are working collaboratively with the EU Policy Board to develop research priorities. In terms of substances, the pesticides group is expected to include:
- Chlorpyrifos
- Dimethoate
- Pyrethroids
- Glyphosate and POE-tallowamine
- Fipronil
Deliverable 4.5 on the 2nd list of HBM4EU priority substances provides a more detailed summary of the rationale for the selection of each substance and group of substances on the 2nd list of HBM4EU Priority Substances. Proposed activities are also outlined in the deliverable 4.5, for consideration by the Chemical Substance Group Leaders.
Disclaimer
The HBM4EU project was launched in 2016 with the aim of improving the collective understanding of human exposure to hazardous chemicals and developing HBM as an exposure assessment method. The project had €74m in funding and jointly implemented by 120 partners from 28 participating countries – 24 EU member states plus Norway, Switzerland, Iceland and Israel and the European Environment Agency. One of its aims was to ensure the sustainability of HBM in the EU beyond 2021. The project ended in June 2022. The website will not be updated any longer, except the page on peer reviewed publications, but will be online until 2032.


