The ranking of substances on the short list was then sent to the HBM4EU Management Board and the EU Policy Board.
EEA and DG RTD engaged in bilateral discussions with the members of the EU Policy Board to better understand the policy rationale behind the nomination of specific substances.
On 5 March 2018, the EU Policy Board and the HBM4EU met separately in order to reflect on the substances on the short list, the ranking and to identify their priorities for action.
At the meeting of the HBM4EU Management Board, EEA presented the ranked list, together with some additional reflections on the policy relevance of each substance group, drawn from the bilateral discussions. Members of the Management Board discussed each of the substances and groups of substances, starting from the first substance ranked on the list according to the global scores. Discussions focused on the relevance and possible interest of including each substances or substance group in the HBM4EU programme.
Certain substances that had been ranked highly were not considered a priority for research under HBM4EU.
- Human biomonitoring was not considered to be the right tool to explore human exposure to nanomaterials (rank 12, 19 and 26). This was because of the lack of analytical methods and the tendency for nanoparticles to conglomerate, so confounding results. Nanomaterials could be considered under work package 16 on emerging substances, but there are currently no available resources in this work package. Nanomaterials were therefore de-prioritised.
- Regarding the cyclic siloxanes (rank 17), experts noted that earlier method development had failed, pointing to the difficulty in avoiding sample contamination. While some joint methods are available, single substances cannot be differentiated. Cyclic siloxanes were therefore de-prioritised.
- Quaternary ammonium compounds (rank 10) were not considered a priority, due to the relatively low hazard score.
Agreement was reached on the substances to be prioritised from the scientific perspective of the Management Board. Suggestions of activities to be undertaken for each substance/group of substances were also discussed.
A meeting of the EU Policy Board ran in parallel with the Management Board meeting. Its members also discussed the ranking and agreed on the priorities from a policy perspective.
On the 6th of March 2018, a bilateral discussion with both members of the Management Board and the EU Policy Board took place in order to agree on the 2nd list of priority substances. Overall, the two boards had chosen the six of the same substances for prioritisation.
The EU Policy Board also prioritised UV filters (benzophenones) and diisocyanates, while the HBM4EU Management Board prioritised arsenic. It was agreed to include these three substance groups.
Substances prioritised by the Management Board and the EU Policy Board
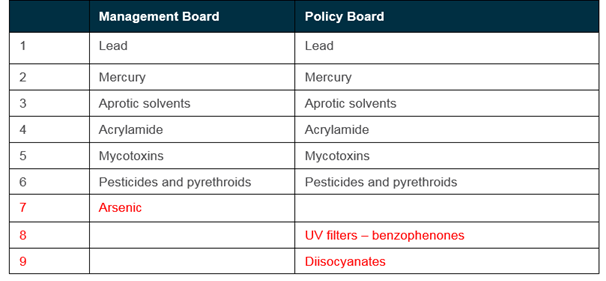
Consensus was reached on the substances to be prioritised for inclusion on the draft 2nd list of HBM4EU Priority Substances, to include all substances in the table above.
The substances included on the 2nd list includes the majority of the top ranking substances emerging from the scoring and ranking of substances under step 7. The outcome therefore validates the approach taken to prioritisation substances, with the systematic ranking of substances based on scientific evidence aligning well with the policy priorities at European level.
Disclaimer
The HBM4EU project was launched in 2016 with the aim of improving the collective understanding of human exposure to hazardous chemicals and developing HBM as an exposure assessment method. The project had €74m in funding and jointly implemented by 120 partners from 28 participating countries – 24 EU member states plus Norway, Switzerland, Iceland and Israel and the European Environment Agency. One of its aims was to ensure the sustainability of HBM in the EU beyond 2021. The project ended in June 2022. The website will not be updated any longer, except the page on peer reviewed publications, but will be online until 2032.


