In order to make the prioritisation process transparent and accountable, a consultation on the background documents, scores and categories was foreseen.
In February 2018, the revised background documents, including the scores and the categories, were sent to the EU Policy Board, the National Hubs and the members of the Stakeholder Forum for comments and feedback.
The level of feedback received is documented in the table below. Feedback was provided in a systematic fashion using a consistent feedback form. The compiled feedback forms are available for download here.
Summary of feedback received for different actors against each substances
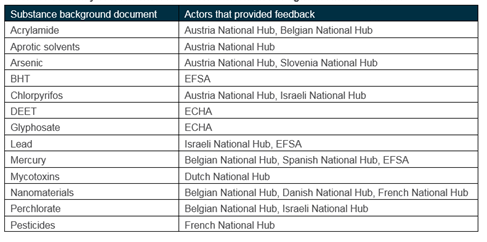
In the majority of cases, actors provided additional information for inclusion in the background document, while agreeing with the scores. Revisions were proposed to the scores for mercury and for glyphosate.
For mercury, the Spanish National Hub proposed a re-evaluation of the scores for elemental mercury versus methyl-mercury, reflecting the fact that methyl-mercury is more hazardous.
For glyphosate, ECHA noted that the ECHA Risk Assessment Committee (RAC) has evaluated glyphosate in the process of harmonised classification and labelling. The RAC opinion[1] states that glyphosate is not carcinogenic. This later evaluation should be used as the basis for the scoring for carcinogenicity and not the earlier IARC conclusion. It was argued that this would more accurately reflect the current knowledge of glyphosate within the EU as not being carcinogenic. In addition, concerning the endocrine activity scoring, EFSA has concluded that glyphosate does not have endocrine disrupting properties based on the available information and no data gaps were identified in the context of this evaluation[2].
The scores for these substances were updated accordingly. It should be noted that for glyphosate, this deviates from the precautionary approach adopted under the prioritisation strategy to base the scoring on the most severe evidence of health effects, with the explicit aim of aligning the evidence base for decision making under HBM4EU with the most recent EU risk assessments.
The final ranking tables are available to download. The final background documents are available for download here.
[1] ECHA RAC (2017) Opinion proposing harmonised classification and labelling at EU level of glyphosate (ISO); N-phosphonomethyl)glycine, ECHA 2017
[2] EFSA (2017) Peer review of the pesticide risk assessment of the potential endocrine disrupting properties of glyphosate, EFSA Journal
Disclaimer
The HBM4EU project was launched in 2016 with the aim of improving the collective understanding of human exposure to hazardous chemicals and developing HBM as an exposure assessment method. The project had €74m in funding and jointly implemented by 120 partners from 28 participating countries – 24 EU member states plus Norway, Switzerland, Iceland and Israel and the European Environment Agency. One of its aims was to ensure the sustainability of HBM in the EU beyond 2021. The project ended in June 2022. The website will not be updated any longer, except the page on peer reviewed publications, but will be online until 2032.


