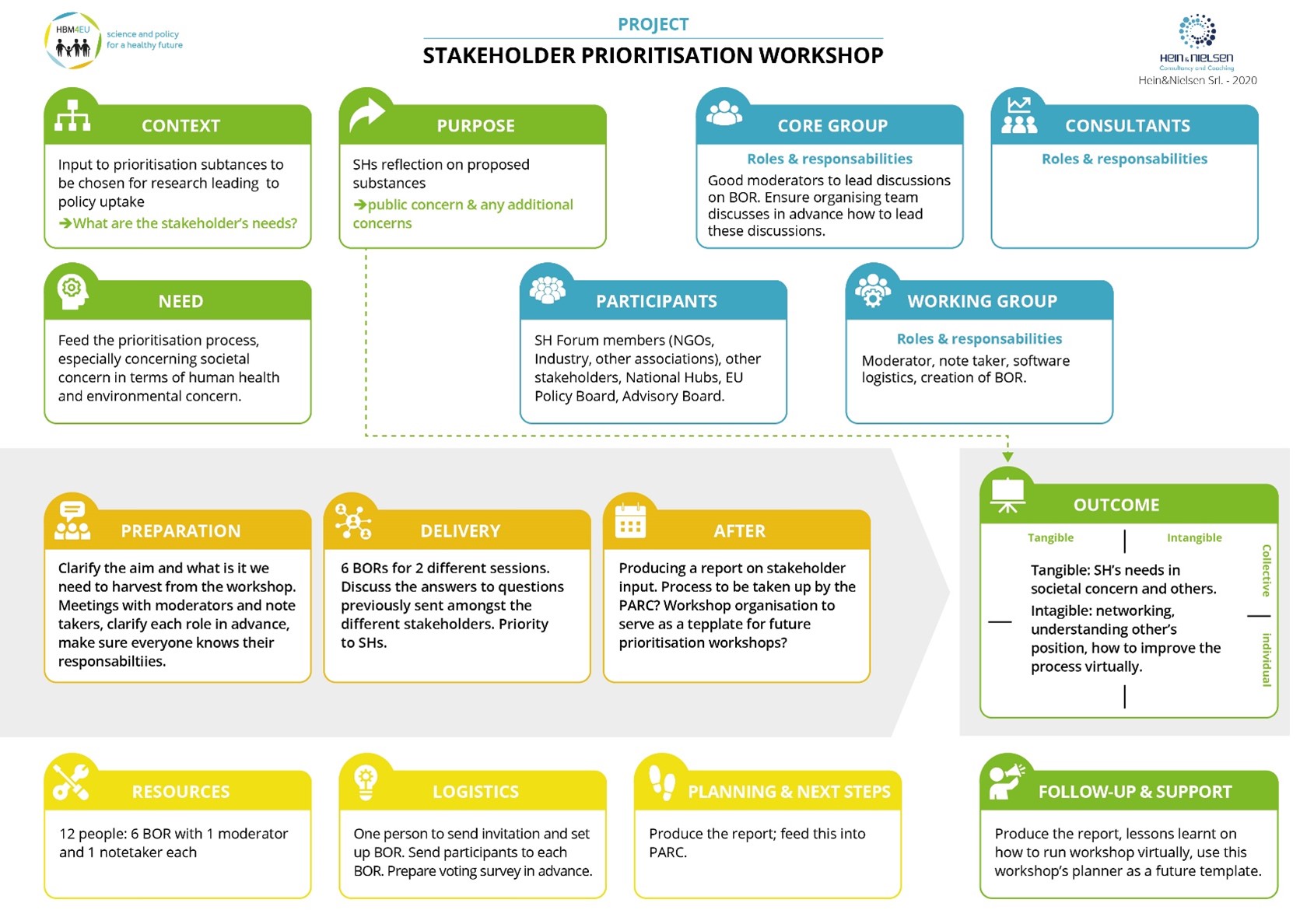
Stakeholder workshop organisation
The aim of the stakeholder workshop was to:
- capture stakeholder reflections on the societal relevance of HBM4EU/PARC;
- discuss priorities for future research under the next partnership (PARC);
- generate new knowledge on the substances on the short list; and
- Understand societal concerns regarding the substances and groups on the short list.
Preparation of the workshop
The stakeholder workshop was held online, due to the restrictions imposed by the pandemic. In order to best prepare this workshop, a plan was laid out and discussed with the task partners (Figure 5). This plan can be used for future workshops.
Two months before the workshop, an invitation was sent out to members of the Stakeholder Forum, the National Hubs, the EU Policy Board and the Advisory Board.
A document was also sent out to the stakeholders with the objectives of the workshop, and a request to select three substances and answer six questions on these substances. The questions were:
- What is the main concern on this substance from a stakeholder perspective?
- Which knowledge gaps should be filled?
- If this is a substance already prioritised under HBM4EU, how can we address issues that have not been covered?
- This list of priority substances will be picked up by the next partnership. How can the PARC address this concern?
- What kind of output/results do you expect from the PARC?
- How would you, as a stakeholder, use the result?
The feedback was used to plan the workshop agenda and prepare discussions.
Plan for the virtual stakeholder workshop
Virtual stakeholder workshop
The workshop took place on the 2nd February 2021, 13h-17h CET, with the agenda provided below. The workshop was held virtually, and the participants (approximately 30) were divided into 6 breakout rooms (BOR) with a moderator and a notetaker in each.
13h Introduction and welcome: 5 min
13h05 EEA: short presentation on the prioritisation and feeding into PARC: 10’
13h15 Questions: 5min
13:15-13h45 Plenary 1: Listening to the members of the stakeholder forum (30min)
13:45-13h50 Voting in the top-3
13h50-14h Short break
14h00-14h05 Organisers to provide a top-10 list of substances for discussion
14h05-15h20 Session 1 in break out room: Discussion on the top 10 ranked substances (1h15)
15h20-15h40 Plenary 2: 20 min
15h40-15h50- short break
15h50-16h50 Session 2 in BOR: Open dialogue about stakeholders’ needs (1h)
16h50 – 17h Thank you and wrap-up.
The discussions in the session 1 and session 2 had as a basis the questions that had been sent to the participants in advance of the workshop.
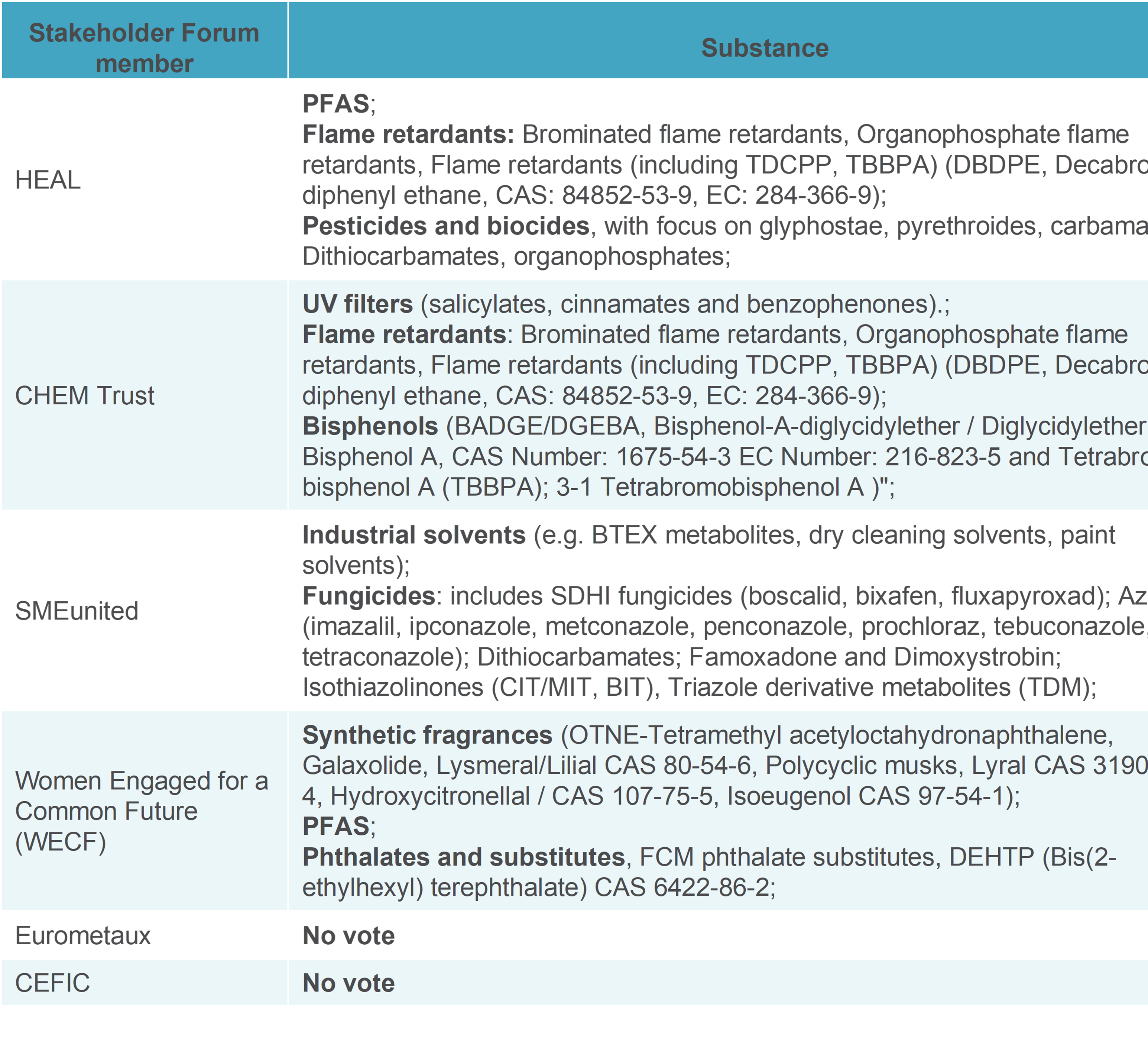
After the 1st plenary session, there was an online voting to identify 10 substances to be the focus of discussion. Each nominating entity was allowed to vote for three substances. The results of the voting are available in the table below. Some substances received the same number of votes and were therefore ranked together.
Since the workshop had the initial aim of harvesting the opinions of the members of the stakeholder forum, please find below the voting of the members of the stakeholder forum members present at the workshop.
There were 3 institutions who did not provide their name; therefore, we could not identify them.
The participants were divided in BORs and this division was previously allocated to ensure a good spread per room of members of the stakeholder forum, national hubs and EU Policy Board. Each BOR had one moderator and one notetaker.
Main conclusions from session 1
In session 1, the discussions focused on the top 10 substances. The questions that helped guide the discussions were:
- What is the main concern on this substance from a stakeholder perspective?
- If this is a substance already prioritised under HBM4EU, how can we address issues that have not been covered?
- What kind of output/results do you expect from the PARC?
From a stakeholder perspective, the need for a specific research question in the context of closing a certain knowledge gap for a better regulatory outcome was mentioned several times. Even though the substance groups are often very wide and can be overlapping, it would be beneficial to identify specific substances within the groups, as well as specific questions and clarity on policy issues.
The different stakeholders’ opinions coming for the discussions held for session 1 are gathered below.
- The relevance of substances/substance groups should be based on high exposure and/or vulnerable groups (e.g. occupational exposure), as well as to the development of capacity and infrastructure/tools (e.g. non/suspect target analysis).
- It is also noteworthy to understand the extent to which the results can be used for regulatory purposes, with special attention given to mixtures, EDCs and occupational exposure.
- Regarding addressing issues that have not been covered in HBM4EU, there should be clarity on what is and what is not covered by PARC, as one cannot do everything. PARC is a research project, which generates information rather than debating decision making policies. PARC is not about challenging regulatory decision processes. It will generate evidence to support certain regulatory processes, but not question the processes themselves. Background documents need to be clear on that, even if the categories are broad.
- For example, on trace metals, what specific questions are we trying to answer in PARC?
- Moreover, in terms of policy issues, discussions on risk management should be held outside the PARC.
- The importance to study different groups of chemicals was also mentioned, with focus within groups on a limited number of substances, and to build up knowledge for data poor substances. Attention was also given to developing Standard Operating Procedures (SOPs) for HBM study designs, to achieve comparability; and to have a better definition of risk assessment tools: from hazard based to risk based.
- One of the overall outputs that is expected from the PARC is better HBM data on substances that are expected to be present in the environment, and guidance on chemicals that are not (yet) regulated but should be. Methodology to be developed can look at relevance, such as the people exposed, production volumes, easy of analyses.
Main conclusions from session 2
In the second session, on the stakeholders’ needs, the questions below were used to steer the discussions:
- Which knowledge gaps should be filled?
- This list of priority substances will be picked up by the next partnership. How can the PARC address this concern?
- How would you, as a stakeholder, use the result?
Question 1: Which knowledge gaps should be filled?
Regarding the knowledge gaps that needed to be filled, the general comments focussed on the attention that needs to be given to the grouping of substances, to ensure that one finer granularity is not lost.
It is also necessary to have clear definitions of knowledge needs prior to activities being undertaken on each substance and depending on the regulatory question, the data needs to be EU wide.
An interest in exposures in specific environments, e.g. industrial/occupational was also pointed out.
In terms of data gaps, variation across Europe should also be addressed as well as a comparison between internal concentration in humans when compared to environmental concentrations which may be indicative of legacy exposure. Supporting information to complement concentration data – age, gender, occupational, socio-economic status, would also be relevant. Methodological rigour will also ensure reliability, and the different results would support the work of different stakeholders who need different types of information. There is also a need to focus on data gaps that need to be filled to guide policy actions.
For emerging chemicals: increase in exposure could serve as early warning and trigger immediate exposure reduction actions to avoid further increase.
An interest in looking for chemicals across matrices, and multi-source monitoring – indoor air, dust – to assess exposure sources was also discussed together with the availability of the data varying depending on the different chemicals.
The need to align HBM activities to complement and not compete with ongoing regulatory processes was also mentioned, as HBM is an add-on to other exposure methods. In a legal context, good exposure data (who and how) is relevant, same for prevention which is becoming more of an issue in occupational settings.
Other themes discussed were:
- How can essential use approach be applied in risk management?
- Safe and Sustainable by Design – development of methods, criteria and processes
- How can HBM data be used in risk assessment processes, in practice?
- Identity, production volumes, uses and metabolites (cf. regulation for pesticides and veterinary drugs, EC 11/86)
- Exposure patterns & levels of workers vs. population
- Risk governance of chemicals for which there is little information: Risk assessment (specific chemicals) vs. risk management (specific and sum/group methods)
- Target substances where exposure is not reversible (long persistence)
Stakeholders are also expecting that the process of generating new scientific evidence is constantly evolving and are hoping for a better understanding of how these chemicals are present in the body.
A strong interlinkage with other projects is also needed to ensure synergies, create a transparent process and a strong interlinkage between stakeholders.
Expectations are also high regarding PARC, in terms of having better tools on the exposure and hazard side to improve regulatory risk assessment in particularly in having good tools to assess emerging risks and its importance to deliver good early warning systems. PARC will go further than HBM4EU and it is important to have continuity in the overarching information, not only HBM but also upstream, sources, how much exposure, what is the kinetic in humans, linking it to the hazard side, getting information from in vitro, and from internal to external exposure and vice versa.
Participants also mentioned that in order to have a mandatory HBM surveillance it is necessary to address ethical concerns and GDPR issues around data use.
Question 2: This list of priority substances will be picked up by the next partnership. How can the PARC address this concern?
Regarding question 2, participants mentioned the following points:
- The need to ensure that substances are addressed coherently across the PARC – both hazard and exposure.
- Testing should be broader and include as many chemicals as possible in as many tissues as possible.
- Use HBM more for early warning and emerging substances was mentioned again for chemicals and their effects on humans (and the environment).
- New data on the same substances is still necessary to evaluate restriction measures and policy efficacy by looking at time-trends.
- For new/unregulated chemicals more research can be performed for less studied chemicals in the group and media (e.g. for PFAS), and use analytical grouping methods to capture more compounds effectively (e.g. sum methods).
- Analytical capacities still need to be strengthened with the need to develop effect biomarkers.
Question 3: How would you, as a stakeholder, use the result?
Under question 3, participants stressed the following points:
- the importance to have data that can be used for regulatory aspects was mentioned as it can feed into risk management decisions, to have relevant information for political decision making on how chemicals affect society.
- For all substances, there is a concern by citizens/organisations who think legislation is not enough to prevent exposure. HBM could address the concern and indicate whether policy actions are needed, and which ones.
- Occupational exposure was also referred to as an inclusion group for large studies.
- It is also necessary to have evidence to strengthen regulatory measures, biological methods, g. in-vitro methods, new biological guidance values for workers and in the general population (and the environment), adverse outcome pathways (AOPs), in-silico methods (QSARs, HTPs, modelling e.g. in support of SSBD) and monitoring of chemicals/effects in vulnerable groups.
- Target information to citizens in general, not only for pregnant women and children, should be more readily available online.
- PARC could use its results to promote the obligation of an assessment method for toxicity of the substances and mixtures under REACH for example, by having a mixture assessment factor for each substance, which is currently being discussed.
Conclusion
The 3rd prioritisation round in HBM4EU produced a short-list of substances for Human Biomonitoring that could be used as a starting point for the PARC, the follow-up initiative to HBM4EU.
The background documents referring to the substances in the short list will be finalised in HBM4EU and provide to the PARC consortium and a valuable information foundation for their work.
The results of the stakeholder workshop, as captured in this document, will also inform the PARC and provide perspective on stakeholder priorities and concerns. From that workshop, main stakeholder needs include:
- Producing evidence to answer targeted policy questions on specific substances;
- Developing and implementing an early warning system for certain substances;
- Tracking policy efficacy using indicators showing time-trends of exposure, both human and environmental;
- Evaluating and improving the use of exposure data in risk assessment;
- Ensuring regulatory uptake of results, in particular in impact assessment for chemical policies;
- Strengthening the occupational surveillance, and organising workplace monitoring of different metals;
- Providing access to trustworthy analytical capacity and results; and
- Supporting the discussion on the value to be taken for the Mixture Assessment Factor (MAF).
Disclaimer
The HBM4EU project was launched in 2016 with the aim of improving the collective understanding of human exposure to hazardous chemicals and developing HBM as an exposure assessment method. The project had €74m in funding and jointly implemented by 120 partners from 28 participating countries – 24 EU member states plus Norway, Switzerland, Iceland and Israel and the European Environment Agency. One of its aims was to ensure the sustainability of HBM in the EU beyond 2021. The project ended in June 2022. The website will not be updated any longer, except the page on peer reviewed publications, but will be online until 2032.