 The HBM4EU scoping document on anilines provides background information on anilines, identifies relevant policy questions on the group of substances and outlines research activities under HBM4EU. The scoping document was produced by Tiina Santonen of the Finnish Institute of Occupational Health in November 2017 and updated in December 2020.
The HBM4EU scoping document on anilines provides background information on anilines, identifies relevant policy questions on the group of substances and outlines research activities under HBM4EU. The scoping document was produced by Tiina Santonen of the Finnish Institute of Occupational Health in November 2017 and updated in December 2020.
Click here to read a report on HBM4EU activities on anilines from 2020.
A 2020 report on the legislative status of anilines in the European Union is available here.
A number of communication products have been developed for Diisocyanates and the Anilines, such as the policy brief, infographic, substance report and video.
Aniline is the simplest member of the primary aromatic amines, in which one or more hydrogen atoms of the benzene ring are replaced by amino (-NH2) group, as shown in the figure below. Derivatives of aniline include a wide variety of different substances. Some of these (like benzidine and MOCA) are composed of two combined aromatic rings. Many aromatic amines may cause methemoglobinemia in humans. Aniline and many of its derivatives are known or suspected human carcinogens. Several aniline derivatives can also cause skin sensitization. Classical members of this family are bladder carcinogens 2-naphtylamine and benzidine, both of which have been restricted in the European Union (EU) implying that there is no exposure to these compounds. A large number of substances in the aniline group are on the market in the EU. Several aniline derivatives can be found also from the list of substances restricted under REACH. Aniline compounds are also formed as degradation products from azo-colourants, pharmaceuticals and from aromatic isocyanates used for polyurethane polymers, lacquers, foams and adhesives. When looking at those aniline substances that are produced or imported in the EU at amounts above 1,000 tonnes per year (tpa) according to the European Chemical Agency’s (ECHA) registration database and that have significant health hazards (other than only irritation/corrosion) the following substances can be retrieved (links take the reader to the substance infocard produced by ECHA): Many anilines have been registered under the REACH Regulation for intermediate use only. These include for example 4-aminoazobenzene, 4-methyl-m-phenylenediamine, 6-methoxy-m-toluidine, 5-nitro-o-toluidine, and 4,4’-methylenedi-o-toluidine. Although also these compounds have serious health hazards, they are not considered further because of the limited exposure due to intermediate use. In contrast, MOCA and MDA are currently authorized under REACH. Both of these chemicals are genotoxic carcinogens to which a threshold for carcinogenic effects cannot be assigned. Both MOCA and MDA are easily absorbed via the skin. Therefore, biomonitoring is the best method for assessing occupational exposure to these substances. MDA is also a degradation product and a metabolite of MDI, one of the Diisocyanates. Following on the Advisory Board’s advice to strengthen the science-policy interface, HBM4EU developed a strategic and systematic approach to outreach and align science and policy. A legislative mapping exercise was done by RPA Consultants, providing relevant public policy processes that may benefit from the knowledge generated under HBM4EU. The documents are available for consultation here, with the tables presented here. Different aniline compounds may exist in various products or be formed as degradation of other products. Exposure may occur e.g. due to the pigment used in various products like hair dyes. Knowledge is needed on the exposure of general public and workers (including professionals like hairdressers) to anilines, the identification of compounds and sources of exposure. In addition, there is a need to identify new biomarkers for anilines. Specific policy questions are: Below, important anilines are discussed in some detail and knowledge needs that can be addressed under HBM4EU are identified. MOCA is mainly used as a curing agent of the polyurethane products. It has a low vapour pressure and it is well absorbed through the skin. Therefore biomonitoring is the best method to assess occupational exposure to MOCA. Exposure to MOCA can be biomonitored by measuring MOCA excreted into the urine (free and conjugated MOCA). These methods are well established and used in occupational surveillance of workers. ECHA has recently made a dose-response analysis for the carcinogenicity of MOCA and calculated cancer risk levels for different urinary MOCA levels measured as total urinary MOCA in the end of the work-shift in the end of the work week (ECHA, 2015a). In addition, the EU Scientific Committee on occupational exposure limits (SCOEL) has recommended a biological guidance value (BGV) for MOCA (SCOEL, 2013). MOCA is listed as a substance of very high concern under the REACH Regulation, and its uses are subject to authorisation. An application for authorisation for the use of MOCA was submitted to ECHA in 2016 (ECHA, 2016a). The request for authorisation covers up to 89 sites in EU using MOCA as a curing agent in polyurethane production. The estimated number of workers exposure to MOCA in the EU is only about 200. Authorization has been applied for 12 years. There is, however, no European Commission (EC) decision nor ECHA’s Risk Assessment Committee (RAC) and Socio-Economic Analysis Committee (SEAC) recommendation on the authorization available yet. The applicant has used biomonitoring data to assess the workers’ exposure to MOCA. In addition, there are established methods available and published studies, especially from UK, on the biomonitoring of MOCA. Since there are substitutes for MOCA available for the use in polyurethane production, the use of MOCA may cease within becoming years when companies are able to move to the substitutes. Therefore, MOCA might not be a very relevant candidate for further studies under HBM4EU although biomonitoring of MOCA would still be needed in EU as long as there are authorized uses in the EU. Furthermore, biomonitoring in workers should reveal a decrease over time (monitoring policy effectiveness). The general population is not exposed to MOCA, and the levels of MOCA and its metabolites in the urine of the general population are below the detection limits. We have sufficient information on the toxicity and occupational exposure to MOCA. Validated biomonitoring methods are available in EU and information for the use of available biomarkers in occupational risk assessment. There is therefore no need for further research actions. The production and use of 4,4’-MDA is authorized under REACH. 4,4’-MDA is easily absorbed through the skin and biomonitoring is therefore the best method to assess occupational exposure. There are well established methods for the biomonitoring of 4,4’-MDA, which are based on the analysis of total urinary MDA excretion. The Risk Assessment Committee (RAC) of ECHA has derived a dose-response for the carcinogenicity of MDA and calculated cancer risk levels for different urinary 4,4’-MDA levels measured as total urinary 4,4’-MDA in the end of the work-shift in the end of the work week (ECHA, 2015b). There are two applications for the authorization of 4,4’-MDA under REACH. They concern: The total number of exposed workers in these uses is 56. The applicant of the authorization provided biomonitoring datasets on the exposure of workers in these uses, and these data were used by RAC in the assessment of excess cancer risk to workers. Due to the limited use (other than intermediate use) and limited number of workers exposed to MDA, occupational exposure to 4,4’-MDA in its industrial use is not a good candidate for further work under HBM4EU. We have sufficient information on the toxicity and occupational exposure to 4,4’-MDA in the industrial use of this substance. Validated biomonitoring methods are available in EU and information for the use of available biomarkers in the risk assessment of occupational MDA exposure. There are only limited numbers of workers using 4,4’-MDA but exposure to 4,4’-MDA formed from methylene diphenyl diisocyanate may occur among the large group of workers and needs further studies (see below on diisocyanates). There is no need for further research actions related to the occupational exposure to 4,4’-MDA in its industrial use. Exposure to MDA in the use of methylene diphenyl diisocyanate (MDI). MDA is one of the degradation products and main metabolites of methylene diphenyldiisocyanate (MDI, CAS 101-68-8). Measurement of urinary MDA can be also used to measure occupational exposure to MDI. Similarly, toluene diamine (2,4-TDA or 2,6-TDA) can be used as a marker for exposure to toluene diisocyanate (TDI, CAS 584-84-9 for 2,4-TDI and 91-08-7 for 2,6-TDI). These diisocyanates are widely used in different applications (e.g. foams, sealants, coatings) throughout the EU, total volume in commerce is 2.5 million tpa (ECHA, 2016b). These diisocyanates (together with non-aromatic hexamethylene diisocyanate, HDI) are important occupational respiratory sensitizers; they are causing several thousand new cases of respiratory allergies (mainly asthma) annually in Europe. 4,4’-MDA (and isomers) is also the major cause of non-compliance of black nylon kitchen utensils imported from China, and the continuous EC testing requirement under the food contact materials legislation EC 10/2011. The source is likely from recycled polyamide (nylon), and from polyamide containing isocyanate lacquers used to coat the glass fibre reinforcement in the utensils. Aromatic isocyanates are also used in adhesives for laminated flexible plastic food packaging. (Mortensen et al. 2005, Trier et al. 2011). Aromatic Polyurethane polymers are also used in medicinal utensils, e.g. for stomi-bags, as nets operated into patients, in blood bags and tubing, as breast implants from where metabolites have been released and measured in the patients’ blood causing sensitisation. The use of the diisocyanates MDI, TDI and HDI has been recently proposed for restriction in the EU, unless specific conditions for workers training and risk management measures apply. The aim of the restriction is not, however, to ban the use of diisocyanates but rather to improve the control of diisocyanate use by obligatory training for good working practices and risk management. Diisocyanate sensitization can occur at very low exposure levels, and sensitive methods to assess exposure e.g. by measurement of diamine levels in urine are still needed in the future. There may be a need to study the possibility to improve the sensitivity of the current diisocyanate monitoring methods, and the effectiveness of the possible restriction on the occupational exposure to diisocyanates. Especially exposure to diisocyanates at small and medium sized enterprises is a concern. There is also a need to better understand the exposure routes of isocyanates, e.g. via air, direct skin contact, or via ingestion of aerosols in order to target risk management measures correctly. In addition, sensitive biomonitoring methods, together with air and skin monitoring methods, are needed for the assessment of the effectiveness of the personal protective equipment. Diisocyanates are an important causes of occupational asthma. Biomonitoring methods available but since asthma may occur at very low exposures, sensitivity of the methods should be high. Some occupational biomonitoring studies are available demonstrating exposure. Knowledge gaps include: Aniline is classified as a suspected carcinogen (carcinogen category 2) under the Classification, Labelling and Packaging Regulation (CLP) in the EU. In addition to the concerns related to the genotoxicity and carcinogenicity, it can cause methemoglobinemia and haemolytic anaemia after long term exposure. Major use of aniline is as an intermediate in the production of different chemicals, including rubber chemicals, dyes, some pesticides, drugs and polyurethane based polymers. It is also used in pH regulators and water treatment products and may also be formed in the thermal degradation of MDI-based polyurethane and reactions in rubber industry. Smoking is also a source of exposure to aniline. The EU risk assessment report from 2008 concludes that there is a need to limit the risk especially for workers but also for the general population near the point sources and consumers due to residues in different products. The main cause of concern is its carcinogenicity and genotoxicity. Toxicity of aniline has been recently assessed also by SCOEL. There are validated biomonitoring methods available for aniline, and SCOEL has recommended a biological limit value based on the measurement of p-aminophenol in urine (SCOEL, 2016). It is also possible to measure aniline itself from the urine or haemoglobin adducts from blood samples. There are some biomonitoring data available both of the general population and workers exposed to aniline. Aniline has not been currently listed as SVHC substance, nor is it subject of any restrictions under REACH. However, it is listed in the PACT-RMOA list under REACH, which includes substances for which a risk management option analysis (RMOA) or an informal hazard assessment for PBT/vPvB (persistent, bioaccumulative and toxic/very persistent and very bioaccumulative) properties or endocrine disruptor properties is either under development or has been completed since the implementation of the SVHC Roadmap commenced in February 2013. The RMOA for aniline was completed in December 2015 and concluded that no action is needed at this time. However, it was noted that the recent exposure data was limited for both workers and for consumers (RIVM, 2015). Further regulatory actions on the aniline could benefit of additional data on both occupational and general population exposure to aniline. A metabolite of aniline, N-acetyl-4-aminophenol, is a commonly used drug, paracetamol, which can cause severe liver toxicity if used at high amounts. Ubiquitous exposure to paracetamol among general population have been demonstrated by Modick et al (2014), with measurable paracetamol levels also detected in the Danish Democophes samples from 2011 (Nielsen et al 2015). The studies from Denmark related self-reported paracetamol intake of the mothers and her reporting of child intake to the biomonitoring of paracetamol among general population, including children and found no clear associations indicating an unknown source (Jensen et al.2014, Nielsen et al 2015, Graungård et al 2016). Methods for the biomonitoring of aniline exist. Toxicity has been evaluated. Some biomonitoring data are available among general population and workers, however, data gaps exists. EU risk assessment concludes that there is a concern for workers, general population and consumers. Knowledge needs include: There are general population biomonitoring data on paracetamol exposure available, mainly from Denmark. Knowledge needs include: o-Toluidine is classified as carcinogenic, category 1B (May cause cancer; H350). It is manufactured and/or imported in the European Economic Area in 10 000 – 100 000 tpa. SCOEL has recently published a recommendation on o-toluidine, which includes also a recommendation for a biological guidance value (SCOEL, 2016). Although there are published methods for the biomonitoring of o-toluidine, limited biomonitoring data is available. The main uses of o-toluidine include its use as a curing agent in epoxy resins and an intermediate in producing azo dyes and pigments, acid-fast dyestuffs, triarylmethane dyes, sulphur dyes, indigo compounds, photographic dyes and synthetic rubber and rubber vulcanising chemicals. The largest use is, however, as an intermediate in the manufacture of herbicides. Earlier it was used in dyes and pigments. o-Toluidine is banned from use in cosmetics under the EU Cosmetics Regulation. The use of azo dyes that release o-toluidine during degradation is not permitted for textiles and other consumer articles in the EU. Still, there are recent reports describing hairdressers exposure to it via the hair waiving products (Johansson et al., 2015). Cherry et al (2011) has estimated that the number of o-toluidine exposed workers in EU is about 5500, mainly in the manufacture of other chemicals. Taking into account that exposure may still occur via hair waiving products, the actual number may be higher. Also general population is exposed to background levels of o-toluidine. Toxicity has been evaluated. Methods for biomonitoring exist but we have limited biomonitoring data for the general population and for workers. Knowledge needs include: p-Toluidine (4-aminotoluene) is manufactured and/or imported in the European Economic Area (1 000 – 10 000 tpa). It is classified as suspected carcinogen (H351). Its main use is in the manufacturing of other chemicals, including dyes, pigments, lubricants and polymer additives. Smoking causes exposure to p-toluidine and it is found in urine in the general population. In hairdressers, no increased exposure to p-toluidine compared to the exposure of general population was seen in a single study (Johansson et al., 2015). Methods for biomonitoring exist and toxicity has been evaluated for p-toluidine. However, we have very limited biomonitoring data among general population and workers. Knowledge needs include: p-Phenylenediamine (CAS 106-50-3) is a common contact allergen present in cosmetics and e.g. in hair dyes and e.g. tattoo inks. It has caused many occupational allergies e.g. among hairdressers exposed due to the contact with hair dyes. It has also been found in black nylon kitchen utensils, like 4,4’-MDA. It has not been regularly biomonitored, although analytical methods for the analysis of it or its metabolites in urine or blood have been published. In these studies exposure of hairdressers to p-PPD has been described. The main hazardous property of p-PDA is its skin sensitizing ability. It has not been listed as SVHC substance, nor is it subject of any restrictions under REACH. However, it has been listed in the PACT-RMOA list under REACH, which includes substances for which a risk management option analysis (RMOA) or an informal hazard assessment for PBT/vPvB (persistent, bioaccumulative and toxic/very persistent and very bioaccumulative) properties or endocrine disruptor properties is either under development or has been completed since the implementation of the SVHC Roadmap commenced in February 2013. In addition, some of the available studies describe potential exposure to other sensitizing aromatic diamines, like 2,5-TDA, m- and p-aminophenols due to the hair dyes. For example, EU Scientific Committee on Cosmetic Products (SCCP, 2007) has concluded that 2,5-TDA is very potent sensitizer and its use in hair dyes cannot be considered safe based on the available data. There are publications on the development of a method to measure exposure to p-PPD and testing of this method in hairdressers. Knowledge needs include: Other substances manufactured/imported in EU >1000 tpa include 1,3-diphenylguanidine (CAS 102-06-7). No biomonitoring studies were found. It is manufactured and/or imported in the European Economic Area in 1 000 – 10 000 tpa. 1,3-diphenylguanidine is used in polymers and manufacturing of rubber and can be released in the environment from many construction, textile, furniture and rubber materials. Few occupational contact allergies have been reported due to 1,3-diphenylguanidine. It is classified as suspected of damaging fertility (H361). It has been subject for substance evaluation under REACH and there are some concerns on its potential genotoxic activity. Another comment raised during the evaluation process relates to the degradation products which may be formed e.g. during rubber manufacturing. These may include e.g. aniline. Anilines manufactured or imported (in commerce) in EU at amounts of 100-1000 tpa include following substances: In the interest of transparency and accountability, HBM4EU invites interested stakeholders to submit comments on the scoping document on anilines. All submitted comments will be made available for download on this webpage and will be taken into consideration by the HBM4EU consortium, where possible. Please click here to submit your comments. Please click here to access the Substance Report. Cherrie JW, Gorman Ng M, Shafrir A and Van Tongeren M, Mistry R, Sobey M, Corden C, Rushton L, Hutchings S (2011a) Health, socio-economic and environmental aspects of possible amendments to the EU Directive on the protection of workers from the risks related to exposure to carcinogens and mutagens at work. o-Toluidine. IOM research project: P937/19, May 2011. ECHA (2015b) Application for authorisation: establishing a reference dose response relationship for carcinogenicity of technical MDA. RAC/32/2015/10 rev 1 ECHA (2016a) Application for authorization by REACH Law Ltd in its legal capacity as Only Representative of Suzhou Xiangyuan Special Fine Chemical Co., Ltd for Industrial use of MOCA as a curing agent/chain extender in cast polyurethane elastomer production ECHA (2016b) Annex XV restriction report. proposal for restriction. Diisocyanates. v. 2.0. 6.10.2016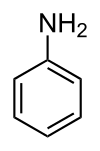
MOCA
Knowledge gaps and HBM4EU research activities on MOCA:
4,4’-MDA
Knowledge gaps and HBM4EU research activities on 4,4′-MDA:
Diisocyanates
Knowledge gaps and HBM4EU research activities on diisocyanates:
Aniline and paracetamol
Knowledge gaps and HBM4EU research activities on aniline:
Knowledge gaps and HBM4EU research activities on paracetamol:
Knowledge gaps and HBM4EU research activities on o-toluidine:
Knowledge gaps and HBM4EU research activities on p-toluidine:
p-PDA
Knowledge gaps and HBM4EU research activities on p-PPD:
Other high production volume (HPV) aniline compounds
For these, no systematic data search have been performed but according to the available information only limited/no biomonitoring data exists for these compounds.
Disclaimer
The HBM4EU project was launched in 2016 with the aim of improving the collective understanding of human exposure to hazardous chemicals and developing HBM as an exposure assessment method. The project had €74m in funding and jointly implemented by 120 partners from 28 participating countries – 24 EU member states plus Norway, Switzerland, Iceland and Israel and the European Environment Agency. One of its aims was to ensure the sustainability of HBM in the EU beyond 2021. The project ended in June 2022. The website will not be updated any longer, except the page on peer reviewed publications, but will be online until 2032.


