The 3rd prioritisation process was run from June 2020 to June 2021 with the aim of feeding into the Partnership for the Assessment of Risk from Chemicals (PARC). This is a partnership proposed under Horizon Europe as a follow-up to HBM4EU, with a broader focus on chemical risk assessment.
The purpose of the PARC is to drive innovation in chemical risk assessment and thereby enable the sustainable use and management of chemicals whilst protecting human health and the environment and contributing to a non-toxic environment by:
- strengthening the scientific basis for chemical risk assessment in the EU, by bringing risk assessors and managers together with scientists to accelerate method development, the generation of necessary data and knowledge, and
- facilitating the transition to next generation evidence-based risk assessment.
For detailed information, please consult the draft concept paper here.
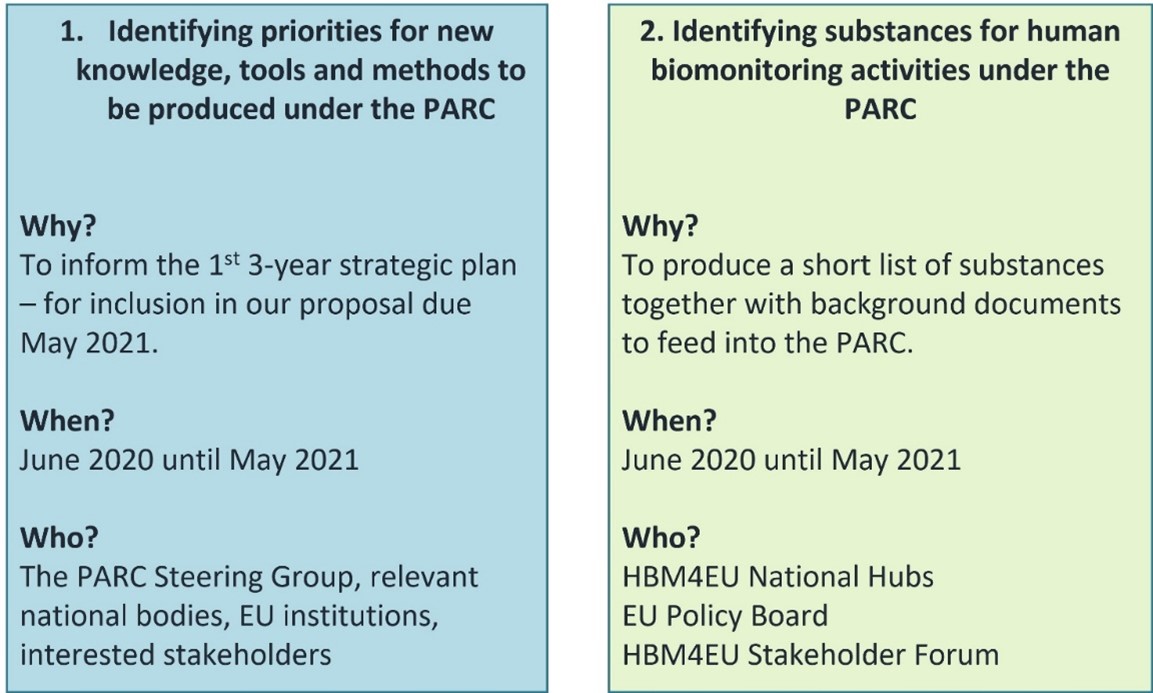
HBM4EU led two parallel prioritisation processes
- To identify priorities for new knowledge, tools and methods on aspects of chemical risk assessment
- To identify a shortlist of substances to be the focus of human biomonitoring activities under the PARC
The objectives and nominating entities involved in the two prioritisation processes are presented below.
Identifying PARC priorities for new knowledge, tools and methods on aspects of chemical risk assessment
HBM4EU conducted an online survey to identify priorities in the following six areas:
- Overall knowledge needs
- Monitoring and exposure
- Hazard assessment
- Innovation in regulatory risk assessment
- Concepts and toolboxes
- Any other priorities
The results of the survey are summarised here. This fed into the development of the proposal for the PARC.
Identifying a shortlist of substances to be the focus of human biomonitoring activities under the PARC:
HBM4EU deliverable D4.8 Third list of HBM4EU priority substances summarises the steps in the 3rd prioritisation process to identify chemicals for human biomonitoring activities. The process involved the following steps:
- An online survey was conducted allowing National Hubs, members of the EU Policy Board and the Stakeholder Forum to nominate substances for Human Biomonitoring (HBM) research. Participants were asked to nominate up to five substances or groups of substances and provide their chemical name and CAS number. They could also nominate chemical mixtures and emerging substances – as groups of substances.
- We produced a long list of all substances nominated by the survey participants. The long list may be found here: Long list HBM for PARC.
- The long list was reduced to a short list of 25 substances, based on substances nominated by the members of the EU Policy Board and those substances nominated by more than one survey participant. The short list of substances may be found in the table below: Short list HBM for PARC.
- We produced background documents on each substance/substance group on the shortlist available to the nominating entities and requested information against the prioritisation criteria, namely hazard properties, exposure characteristics, regulatory status, public concern and technical feasibility.
- We organised a stakeholder workshop to gather input from the members of the stakeholder forum and a broader range of stakeholders on their concerns regarding substances on the short list. The results of the workshop are captured in Deliverable 4.10 2nd Report on stakeholder consultation and mapping of needs.
Disclaimer
The HBM4EU project was launched in 2016 with the aim of improving the collective understanding of human exposure to hazardous chemicals and developing HBM as an exposure assessment method. The project had €74m in funding and jointly implemented by 120 partners from 28 participating countries – 24 EU member states plus Norway, Switzerland, Iceland and Israel and the European Environment Agency. One of its aims was to ensure the sustainability of HBM in the EU beyond 2021. The project ended in June 2022. The website will not be updated any longer, except the page on peer reviewed publications, but will be online until 2032.